Links to prescribing information and adverse event reporting information can be found at the bottom of the page. For UK Healthcare professionals only.
Clinical Evidence
Vectibix® is recommended by NICE for previously untreated RAS wild-type mCRC in combination with FOLFOX or FOLFIRI1
PRIME STUDY: Vectibix® + FOLFOX4 for first-line treatment of mCRC2
- The first phase 3 study to evaluate outcomes by RAS status in first-line2
- A pre-defined retrospective analysis assessed the effect of Vectibix® + FOLFOX4 versus FOLFOX4 on outcomes among patients with wild-type RAS mCRC3
- Primary endpoint: progession-free survival3
- Secondary endpoints: overall survival and safety3
- RAS ascertainment rate was 90% (1060 patients)3
- RAS analysis was exploratory3
Study design
PRIME was a multicentre, randomised study evaluating the efficacy and safety of Vectibix® + FOLFOX4 versus FOLFOX4 alone in patients with previously untreated mCRC.2
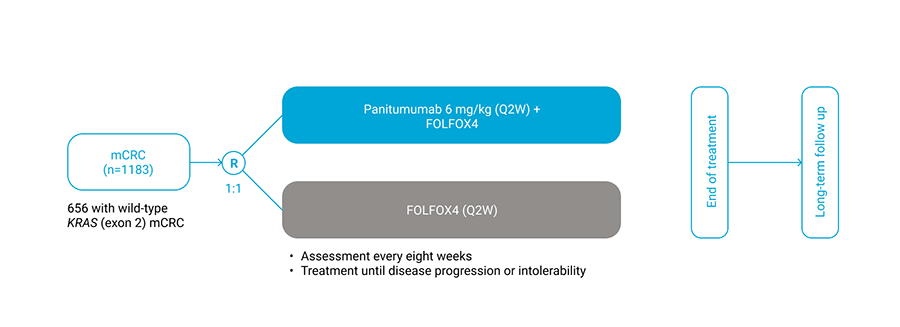
Results of the clinical trial
Among 512 patients without RAS mutations, patients treated with panitumumab + FOLFOX4 had a greater median progression-free survival time than patients treated with FOLFOX4 alone3:

On average, patients in the panitumumab + FOLFOX4 group had longer overall survival time than patients treated with FOLFOX4 alone3:

All p-values are descriptive.
PRIME study: WT KRAS mCRC safety analysis set4
AEs (grade 3/4)
| WT KRAS (n = 649) | |||
|---|---|---|---|
| AE, % |
Panitumumab + FOLFOX4 (n = 322) |
FOLFOX4 (n = 327) |
|
| Neutropenia | 43 | 42 | |
| Skin toxicity | 37 | 2 | |
| Diarrhoea | 18 | 9 | |
| Neurologic toxicities | 16 | 16 | |
| Hypokalaemia | 10 | 5 | |
| Fatigue | 10 | 3 | |
| Mucositis*† | 9 | < 1 | |
| Hypomagnesaemia | 7 | < 1 | |
| Paronychia† | 3 | 0 | |
| Pulmonary embolism | 3 | 2 | |
| Febrile neutopenia | 2 | 2 | |
|
Infusion-related reaction (panitumumab)†︎ |
< 1 | - | |
*Includes preferred terms: stomatitis, mucosal inflammation, aphthous stomatitis, mouth ulceration, mucosal dryness, and mucosal ulceration; †No grade 4
STUDIES OF CETUXIMAB AND PANITUMUMAB IN COMBINATION WITH FOLFIRI FOR mCRC
Study 314: Vectibix® + FOLFIRI for first-line treatment of mCRC5,6
- Phase II single-arm study to evaluate first-line panitumumab plus FOLFIRI* in patients with mCRC5
- n=68 RAS WT received first-line panitumumab + FOLFIRI every 14 days5
- Primary endpoint: objective response rate (ORR)5,6
- Secondary endpoints: duration of response, progression-free survival (PFS), time to progression, disease control rate, safety5
- RAS analysis was exploratory6
*FOLFIRI regimen (irinotecan 180 mg/m2; leucovorin 400 mg/m2; fluorouracil bolus 400 mg/m2, then 46-hour infusion 2400–3000 mg/m2)
CRYSTAL: FOLFIRI + cetuximab for first-line treatment of mCRC7,8
- Phase III open-label, randomised controlled trial to evaluate outcomes for FOLFIRI† + cetuximab vs FOLFIRI† alone in mCRC7
- 599 patients received FOLFIRI† + cetuximab and 599 received FOLFIRI† alone7
- 178 RAS WT patients in the FOLFIRI† + cetuximab weekly arm8
- Primary endpoint: progression-free survival7,8
- Secondary endpoint: overall survival (OS)7
- Post hoc retrospective analysis of RAS WT patients8
†FOLFIRI regimen (irinotecan 180 mg/m2 and leucovorin 400 mg/m2; fluorouracil bolus 400 mg/m2, then 46-hour infusion 2400 mg/m2)
FIRE-3: FOLFIRI + cetuximab for first-line treatment of mCRC9,10
- Phase III open-label, randomised controlled trial to evaluate outcomes for FOLFIRI* + cetuximab vs FOLFIRI† + bevacizumab in mCRC9
- 297 patients received FOLFIRI* + cetuximab and 295 received FOLFIRI† + bevacizumab9
- n = 199 RAS WT in the FOLFIRI* + cetuximab weekly arm10
- Primary endpoint: objective response9,10
- Secondary endpoints: progression-free survival (PFS), overall survival (OS), depth of remission, secondary resection of liver metastases with curative intent, safety and tolerability9,10
*FOLFIRI regimen (irinotecan 180 mg/m2 and leucovorin 400 mg/m2; fluorouracil bolus 400 mg/m2, then 46-hour infusion 2400 mg/m2)
†FOLFIRI regimen (irinotecan 180 mg/m2 and leucovorin 400 mg/m2; fluorouracil bolus 400 mg/m2, then 46-hour infusion 2400–3000 mg/m2)
Key inclusion/exclusion criteria
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Study 3145,6 |
≥ 18 years of age
|
|
| CRYSTAL7,8 |
≥ 18 years of age
|
|
| FIRE-39,10 |
18-75 years
|
|
Demographics*
| Study 3145,6 | CRYSTAL7,8 | FIRE-39,10 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex, male | 80% | 61% | 73% | ||||||||
| Median age | 62 | 60 | 64 | ||||||||
| ECOG status |
|
|
|
||||||||
| Primary tumour |
Colon – 58% Rectum – 42% |
Colon – 60% Rectum – 38% Colon or rectum – 2% |
Colon – 60% Rectum – 36% Colon or rectum – 4% Unknown – 1% |
||||||||
| Metastatic sites |
Liver only – 38% Liver and other – 48% Other only – 14% |
Liver only – 24% At one or two sites – 88% |
Liver – 85% Liver only – 36% Liver not affected – 14% |
||||||||
| Previous treatment | Adjuvant - NR | Adjuvant – 22.5% |
Surgery – 86% Adjuvant – 19% Radiotherapy – 12% |
||||||||
| Ethnicity | White – 96% | ||||||||||
| Other | Number of metastatic sites: |
Tumour location: left – 80% right – 19% |
|||||||||
|
*The demographics presented are for the EGFR-treated arm of each study, prior to the RAS analysis, as some of the studies did not report the demographics of the RAS WT population
RAS testing and endpoints
| Study 3145,6 | CRYSTAL7,8 | FIRE-39,10 | |
|---|---|---|---|
| RAS testing |
Tumour mutational status assessed using DxS kit that utilised allele-specific, real-time PCR
|
Tumour mutations assessed using PCR clamping and melting curve method
≥ 5% mutant allele cut-off was used to call mutations
|
Tumour mutations assessed using pyrosequencing
|
| Endpoints (original study) |
KRAS WT patients
|
All mCRC patients
|
KRAS WT patients
|
| RAS WT analysis endpoints | Exploratory RAS status analysis |
Post hoc retrospective analysis of RAS WT patients
|
Post hoc exploratory analysis of RAS WT patients
|
Clinical trial results
| Outcomes (WT RAS) | |||
|---|---|---|---|
| ORR, % | PFS, months | OS, months | |
| FOLFIRI + panitumumab | |||
| Study 3145,6 | 59.0 | 11.2 | NR |
| FOLFIRI + cetuximab | |||
| CRYSTAL7,8 | 66.3 | 11.4 | 28.4 |
| FIRE-39,10 | 65.3 | 10.3 | 33.1 |
Abbreviations
AE, adverse event; CI, confidence interval; BORR, best overall confirmed response rate; DCR, disease control ratio; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; HR, hazard ratio; IBD,
inflammatory bowel disease; mCRC, metastatic colorectal cancer; MI, myocardial infarction; NGS, next generation sequencing; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free
survival; PCR, polymerase chain reaction; RECIST, Response Evaluation Criteria in Solid Tumours; WT, wild-type.
References
- NICE. Cetuximab and panitumumab for previously untreated metastatic colorectal cancer (TA439).
- Douillard J-Y et al. Journal of Clinical Oncology 2010;31: 4697–4705.
- Douillard J-Y et al. N Engl J Med 2013;369:1023–1034.
- Douillard JY et al. Ann Oncol 2014: 25: 1346–55.
- Köhne CH et al. J Cancer Res Clin Oncol 2012;138:65-72.
- Karthaus M et al. Br J Cancer 2016;115:1215-1222.
- Van Cutsem E et al. N Engl J Med 2009;360:1408–1417.
- Van Cutsem E et al. J Clin Oncol 2015;33:692–700.
- Heinemann V et al. Lancet Oncol 2014;15:1065–1075.
- Stintzing S et al. Lancet Oncol 2016;17:1426–1434.
